Talk on Breastfeeding & Prevention at Happy Mama Expo During Breast Cancer Awareness Month
DroneSense Announces General Availability of Drone as First Responder Solution for Public Safety
AUSTIN, Texas - October 11, 2022 - (Newswire.com)
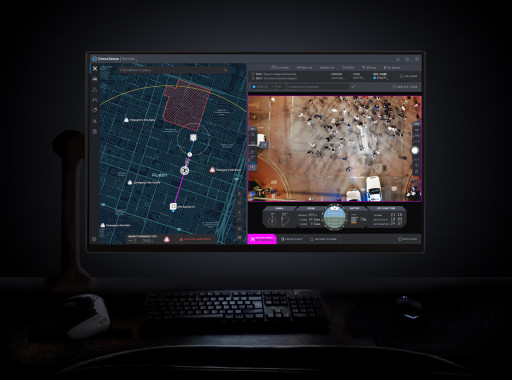
DroneSense (www.dronesense.com), developers of the most trusted and reliable software platform for public safety drone operations, today announced the general availability of DroneSense Remote and Axon Air Remote powered by DroneSense. This breakthrough technology allows public safety agencies to remotely operate and deploy drones in emergencies, giving first responders a real-time view of what is happening before they arrive at the scene.
Many public safety agencies have drone programs where officers or firefighters operate a drone on scene while responding to an incident. Drone as a First Responder (DFR) programs go further, providing vital situational awareness on calls for service even before personnel arrives on scene. This approach protects both communities and public safety by giving agencies more time and information to determine the most appropriate response to a situation. The system retains detailed records of every flight, so that agencies can easily maintain transparency and accountability regarding their use of the technology within the communities they serve.
Agencies looking to start a DFR program now have everything they need for remote operations, thanks to DroneSense and Axon Air powered by DroneSense. DroneSense Remote and Axon Air Remote allow agencies to respond to any 911 call within seconds, with a single mouse click. The platform enables a teleoperator to remotely pilot drones from anywhere and aims to improve tactical decision-making and improve efficiency with fewer officer dispatches. Additionally, Axon Air Remote works seamlessly with Axon Evidence, Axon's digital evidence management system, and Axon Respond, Axon's real-time operations solution.
"We are excited to offer first responders the most advanced and comprehensive remote flying experience through DroneSense and Axon Air," said Chris Eyhorn, CEO of DroneSense. "DroneSense Remote is leading the industry with innovation and support for more drone models than any other platform, allowing agencies of all sizes and budgets to increase officer safety, improve incident response time, and achieve better outcomes."
With thousands of operational flights across leading agencies over the past few months, DroneSense Remote and Axon Air Remote have set a new bar for speed and precision in public safety remote drone operations and established an exciting new option for modern policing. The platform allows agencies to create fully-customizable flights to fit their needs, including flying DFR missions Beyond Visual Line of Sight (BVLOS) under an FAA waiver or remaining within Visual Line of Sight (VLOS) under an existing Certificate of Authorizations (COA) or Part 107 regulations. Built-in preflight checks, simple user interface, customizable geofences, continuous network integrity monitoring, and emergency landing sites create the industry's safest approach for demanding operational environments. DroneSense Remote and Axon Air Remote even adjust for elevated takeoff locations to ensure strict adherence to designated flight altitudes.
"DroneSense Remote is the platform that has enhanced our drone program," said Sgt. Daniel Anders with the San Antonio Police Department. "We now utilize drones to respond to calls for service much faster than we can with other assets."
DroneSense Remote is available for the DroneSense platform, and Axon Air Remote is available for the Axon Air powered by DroneSense platform. DroneSense and Axon Air Remote applications work with public safety's most popular drones, including the DJI Matrice 300, Mavic 2 Enterprise Dual and Zoom, and DJI's new Matrice 30 and Mavic 3 Enterprise series drones.
To learn more about DroneSense Remote, please visit www.dronesense.com/remote or for Axon Air Remote, please visit www.axon.com/products/axon-air.
Contact Information:
Ryan Bracken
Chief Product Officer
[email protected]
512-582-0444
Related Images
Press Release Service by Newswire.com
Original Source: DroneSense Announces General Availability of Drone as First Responder Solution for Public Safety
PetMedic Urgent Care Vet Clinic to Open First Virginia Location
ZetrOZ Systems’ sam® Ultrasound Technology Expands Clinical Education on Sustained Acoustic Medicine
TRUMBULL, Conn. - October 10, 2022 - (Newswire.com)
ZetrOZ Systems, creator and manufacturer of the sam® wearable ultrasound device product line, is continuing to expand its clinical education program on the benefits of sustained acoustic medicine for soft tissue healing, the usage of the pain relief treatment and attaining health insurance coverage of the pain relief device.
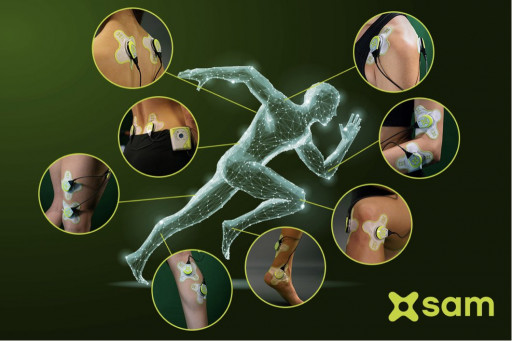
Sustained Acoustic Medicine (sam®) is the only home-use bioregenerative medical device cleared by the U.S. Food & Drug Administration for the treatment of select medical indications, most commonly for chronic joint pain of the shoulder, knee and elbow, and the treatment of overuse injuries. More than 50 clinical and scientific studies support its effectiveness in healing injuries, restoring function and returning patients to work and everyday activities.
"Our sam® technology is touching thousands of lives daily, and we want to continue providing education to more healthcare providers as a way to bring this innovative intervention forward to Americans who can benefit from this treatment," said Dr. George Lewis, founder and CEO of ZetrOZ Systems. "This technology offers results without the need for opioids or other drugs while treating and healing patients up to 40% faster than standard methodologies."
Clinical education on sam® is directed by Dr. Rod Walters and Dr. Rajiv Mallipudi. Walters is a nationally recognized leader in athletic training with decades of experience at major universities and as a consultant to clients including the National Football League, the Big 12 Athletic Conference and Cirque du Soleil. He was inducted into the National Athletic Trainers Association Hall of Fame in 2005.
Dr. Mallipudi, MD, M.H.S, is a board-certified internal medicine hospitalist physician and assistant clinical professor at the Yale School of Medicine in New Haven, Conn. He is an active clinical researcher on healthcare quality improvement, with projects examining methods to reduce length of stay for hospital patients and to improve patient outcomes.
Walters and Mallipudi have a shared goal of educating physicians, physical therapists and athletic trainers about the benefits and use of the sam® device, in hopes of reducing the need for surgeries and pain medication and improving patients' likelihood of returning to work, sports and normal daily activities. By providing clinical training resources, including online videos, medical professionals now have information on best practices for the drug-free and non-invasive treatment of patients' injuries.
Adherence to a sam® regimen across the repair phase provides a necessary and safe intervention to ensure patients avoid chronic, recurring injuries. Recommended practices are specified in the education materials and include:
- The sam® treatment can be applied with one or two ultrasound stimulators.
- The sam® device should be applied a minimum of four times a week.
- Treatments should be applied incrementally to assess patients' response to treatment, from an initial one-hour treatment to a maximum of four hours per treatment.
- For acute conditions, treatment duration can range from 4 to 8 weeks.
- For chronic conditions, treatment can range from 2 to 12 weeks.
Clinical education instructs physicians that sam® can serve as a stand-alone treatment or be used to supplement other treatment approaches to stimulate soft-tissue repair and that its value is as a treatment option that does not require surgery or pain relief medications.
The sam® treatment also is a cost-effective treatment option. For example, a 2020 study on knee osteoarthritis, published in the Journal of Orthopaedic Surgery and Research, found sam® provided a greater improvement in functional effectiveness per dollar of care than the standard care options.
ZetrOZ Systems continues to provide education on sam® technology, most recently participating in the annual PHATS (Professional Hockey Athletic Trainers Society) SPHEM (Society of Professional Hockey Equipment Managers) 2022 Conference in June. Findings showed sam® is a non-invasive, non-narcotic alternative to pain, and that the device's ease of use led athletes to return to normal activity after treatment.
To learn more, visit zetrozsystems.com.
About ZetrOZ Systems
ZetrOZ Systems is leading healing innovation in sports medicine, developing wearable bioelectronic devices for the delivery of sustained acoustic medicine (sam®). Researched and funded by the federal government, ZetrOZ is built on proprietary medical technology of +46 patents and is the exclusive manufacturer and developer of sam®, a product line designed for the treatment of acute and chronic musculoskeletal conditions. To learn more, visit zetrozsystems.com.
Contact Information:
Buse Kayar
[email protected]
Press Release Service by Newswire.com
Original Source: ZetrOZ Systems' sam® Ultrasound Technology Expands Clinical Education on Sustained Acoustic Medicine
KND Labs Earns NSF Certification
Novel Beauty-From-Within Ingredient Lustriva® is Self-Affirmed as GRAS
Wellness Ambassadors Take Charge of Mental Health in Their Communities
PA Options for Wellness’ Medical Marijuana Presence Grows to Six ‘Vytal Options’ Dispensary Locations...
HARRISBURG, Pa. - October 10, 2022 - (Newswire.com)
PA Options for Wellness ("PA Options for Wellness", DBA "Vytal Options" or the "Company"), a leading provider of medical cannabis products in the Pennsylvania medical cannabis market, announced today the opening of two new medical marijuana dispensaries, bringing their retail footprint to a total of six locations throughout Pennsylvania.
The first of the two new dispensaries to open will be Kennett Square, located at 716 West Baltimore Pike in Delaware County, PA. The Kennett Square location will open to patients on Monday, Oct. 17. The second location, located in State College, at 1653 North Atherton Street, will open to patients on Monday, Oct. 31. Both locations will feature on-site consultations, curbside delivery and an interactive pre-order menu with each dispensary open six days a week, from 10 a.m. to 8 p.m.
"Vytal Options, a PA Options for Wellness medical marijuana dispensary, is excited to bring our excellent patient service as well as quality products to the State College and Kennett Square areas," CEO and founder Thomas A. Trite said in a statement. "We were one of the first three medical marijuana Clinical Registrants approved by the Pennsylvania Department of Health to conduct research. After undergoing an extensive review process, we were selected by Penn State University and their College of Medicine to collaborate and research medical marijuana."
To celebrate the grand openings, each location will kick off with a ribbon cutting ceremony at 9 a.m. ET, with members of the Chamber of Business, Penn State Research, and Balanced Veterans Network. A partnership with grower/processer FarmaceuticalRX has also been developed to support the dispensary openings. A proprietary strain "Keystone OG" was created with the processing team at FarmaceuticalRX along with members of Vytal Options marketing and product development teams. The first 100 patients at each location will receive an exclusive, limited edition "Keystone OG" t-shirt to celebrate the collaboration. In addition, many of the state's top Medical Marijuana Grower Processers will be on hand at both locations, educating patients and handing out promotional materials. In addition, PA Options for Wellness will commemorate its latest dispensary openings by making a charitable contribution to organizations within the State College and Kennett Square communities.
PA Options for Wellness proudly offers a curated selection of products across it's brand portfolio, which includes byVytal, Mood, and Solventless. Patients can choose from a wide array of premium cannabis products, including full-spectrum RSO, access to a library of flower strains, solventless rosins, and their signature Troche sublingual. Vytal Options dispensaries will also offer top products from other highly requested brands in the Pennsylvania Medical Marijuana marketplace.
"Pennsylvania has become one of the fastest growing medical markets in the nation and we are proud to be able to widen access to patients across the state," said Thomas A. Trite, Chief Executive Officer of PA Options for Wellness. "PA Options for Wellness will bring top-quality, innovative products to the medical market, meeting the demand of our patients while adhering to strict ethical standards. Providing therapeutic solutions, we focus on combining plant science and medicine to transform the lives of our patients."
In addition to PA Options for Wellness' newest dispensary locations, the Company currently serves patients in the following areas: Harrisburg, Lancaster, Lansdale, and the Lehigh Valley.
About PA Options for Wellness
PA Options for Wellness was founded by Thomas A. Trite, PD, FASCP, in 2014 with the goal of creating a premier medical cannabis service model and becoming the leader in the emerging medical cannabis industry and cannabis research.
Headquartered in Harrisburg, PA Options for Wellness' mission is to be the preferred provider of medical cannabis to qualified, approved patients through high-quality products, and dignified, professional service. The company is focused on research, patient outcomes and quality of life.
PA Options for Wellness is proud to have been awarded one of the first Pennsylvania Clinical Registrant licenses in June 2019 in collaboration with the Penn State College of Medicine. PA Options for Wellness also has a 65,000-square-foot grow/process facility that includes lab space and is located in Duncannon, Perry County.
PA Options for Wellness will bring top-quality, innovative products to the medical market, meeting the demand of our patients while adhering to strict ethical standards. Providing therapeutic solutions, we focus on combining plant science and medicine to transform the lives of our patients.
Media Inquires:
Samantha A. Alderfer
Director of Marketing & Business Development
PA Options for Wellness, Inc.
[email protected]
717-418-4362
Contact Information:
Samantha Alderfer
Director of Marketing & Business Development
[email protected]
Press Release Service by Newswire.com
Original Source: PA Options for Wellness' Medical Marijuana Presence Grows to Six 'Vytal Options' Dispensary Locations in Pennsylvania
Gb Sciences Co-Publishes Study Demonstrating Efficacy of Its Proprietary Cannabinoid Mixtures for the Treatment...
LAS VEGAS - October 10, 2022 - (Newswire.com)
Gb Sciences, Inc. (OTCQB:GBLX), a leading plant-inspired, biopharmaceutical research and drug development company, has co-published a study in the journal Frontiers in Pharmacology that demonstrates the efficacy of its proprietary cannabinoid-containing, minimum essential mixtures (MEM) for the treatment of Parkinson's disease (PD). There is a great clinical need for improved therapeutics, and the market for Parkinson's disease treatments is expected to grow to $8.8 billion by 2026.
"Our drug discovery process has identified promising ratio-controlled mixtures of cannabis-inspired compounds for the treatment of Parkinson's disease, which have proven effective at reducing Parkinsonian motor symptoms in an animal model of the disease," said Dr. Andrea Small Howard, President, Chief Science Officer, and Director of Gb Sciences. "This study allows us to continue addressing unmet clinical needs through the development of novel plant-inspired drugs, and positions Gb Sciences as a contributor to the expanding world of novel PD therapeutics."
For this Gb Sciences-sponsored study, the discovery research using cell models of Parkinson's disease was performed at Chaminade University (Honolulu, Hawai'i, USA), and the MEM refinement and validation research was performed in a zebrafish model of Parkinson's disease at the National Research Council of Canada (NRC). The study entitled "Identification of minimum essential therapeutic mixtures from Cannabis plant extracts by screening in cell and animal models of Parkinson's disease" was co-authored by Gb Sciences' own President and Chief Science Officer Andrea Small-Howard and her collaborators Michael G. Morash, Jessica Nixon, and Lee Ellis from the National Research Council of Canada; Lori M.N. Shimoda and Helen Turner from Chaminade University of Honolulu; and Alexander J. Stokes from the University of Hawai'i at Manoa (Manoa, Hawai'i, USA).
The sequentially reductionist process implemented in this multi-site study preserves some of the entourage-like effects of whole plant extracts while achieving "relative" simplicity within MEM that is a requirement for obtaining the manufacturing and quality control advantages of single-ingredient drugs. This paper identifies promising minimal essential mixtures of cannabinoids based on a step-wise, strategic approach to reducing the complexity of the plant secondary metabolome. The sequential use of in silico, in vitro, and medium throughput in vivo experimental systems has generated refined, de-risked mixtures that can now be tested in additional, higher-cost, preclinical model systems of PD.
Gb Sciences' Parkinson's therapeutics are currently being tested in a rodent model of Parkinson's disease at the University of Lethbridge, and these Parkinson's MEM have been formulated as Oral Dissolving Tablets in preparation for human clinical trials. The Company is preparing for the final rounds of toxicology and pharmacology testing as required for filing an Investigational New Drug (IND) Application with the U.S. Food and Drug Administration.
To learn more about Gb Sciences, visit www.gbsciences.com.
About Gb Sciences and GbS Global Biopharma
Gb Sciences, Inc. is a plant-inspired, biopharmaceutical research and development company creating patented, disease-targeted formulations of cannabis- and other plant-inspired therapeutic mixtures for the prescription drug market through its Canadian subsidiary GbS Global Biopharma, Inc. The "plant-inspired" active ingredients in its therapeutic mixtures are synthetic homologues identical to the original plant compounds but produced under current Good Manufacturing Practices. Gb Sciences' intellectual property portfolio contains six issued U.S. and three issued foreign patents, as well as 17 U.S. and 51 foreign patent-pending applications. In its drug development pipeline, Gb Sciences has five preclinical phase product development programs. Gb Sciences' lead program for Parkinson's disease is being prepared for a first-in-human clinical trial. Gb Sciences' formulations for chronic pain, anxiety, and depression are currently in preclinical animal studies with researchers at the National Research Council of Canada. The company also received positive preclinical proof-of-concept data supporting its complex mixtures for the treatment of Cytokine Release Syndrome, and its lead candidates will be optimized based on late-stage preclinical studies at Michigan State University. Gb Sciences' productive research and development network includes distinguished universities, hospitals, and Contract Research Organizations. To learn more, visit www.gbsciences.com.
Forward-Looking Statements
This press release may contain statements relating to future results or events, which are forward-looking statements. Words such as "expects," "intends," "plans," "may," "could," "should," "anticipates," "likely," "believes" and words of similar import may identify forward-looking statements. These statements are not historical facts, but instead represent only the Company's belief regarding future events, many of which, by their nature, are inherently uncertain and outside of the Company's control. It is possible that the Company's actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward-looking statements. Further, information concerning the Company and its business, including factors that potentially could materially affect the Company's business and financial and other results, are contained in the Company's filings with the Securities and Exchange Commission, available at www.sec.gov. All forward-looking statements included in this press release are made only as of the date of this press release, and we do not undertake any obligation to publicly update or correct any forward-looking statements to reflect events or circumstances that subsequently occur or of which we hereafter become aware.
Contact Information:
Madeleine Moench
[email protected]
Press Release Service by Newswire.com
Original Source: Gb Sciences Co-Publishes Study Demonstrating Efficacy of Its Proprietary Cannabinoid Mixtures for the Treatment of Parkinson's Disease
The Most Expensive reality show bids farewell to Marbella
 Debbie Wingham and her Gossip Stone TV show The Most Expensive say farewell to Marbella at the Hard Rock Hotel, home of Europe’s jet set elites. Debbie Wingham is making the transition from the upscale Spanish town of Marbella to her new home in Dubai and doing it in style with the release of the […]
Debbie Wingham and her Gossip Stone TV show The Most Expensive say farewell to Marbella at the Hard Rock Hotel, home of Europe’s jet set elites. Debbie Wingham is making the transition from the upscale Spanish town of Marbella to her new home in Dubai and doing it in style with the release of the […]