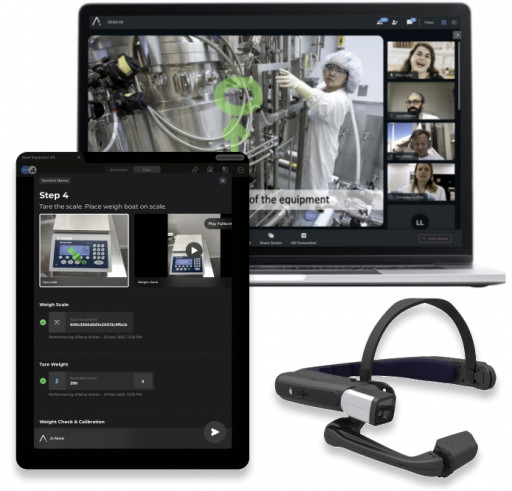
Apprentice Introduces Augmented Work Instructions (AWI) to Help Life Science Organizations Kickstart Their Digital...
TODAY: Gb Sciences Describes Drug Development Progress in Pharma’s Almanac Journal Article
LAS VEGAS - September 6, 2022 - (Newswire.com)
Gb Sciences, Inc. (OTCQB:GBLX), a leading plant-inspired, biopharmaceutical research and drug development company, describes their progress towards achieving significant milestones in their drug development pipeline in an article published in the journal Pharma's Almanac today at https://www.pharmasalmanac.com/articles/unlocking-the-therapeutic-potential-of-plant-inspired-minimum-essential-mixtures.
"Gb Sciences is focusing considerable resources on bringing our lead Parkinson's disease formulations to the clinic with promising results already obtained during the formulation of Oral Dissolving Tablets containing our active ingredients for use in human clinical trials," said Dr. Andrea Small-Howard, President, Chief Science Officer, and Director of Gb Sciences. "We have new data validating the efficacy of our novel anxiety formulations in an animal model, as well as new data demonstrating the improved stability of our oral nanoparticle-encapsulated chronic pain formulations based on newly-adopted manufacturing control processes."
The full article entitled "Unlocking the Therapeutic Potential of Plant-Inspired Minimum Essential Mixtures" also provides some context for their recent drug development successes by discussing Gb Sciences' evolution from a cannabis company to a plant-inspired biopharmaceutical company and what makes their drug development approach truly unique. With conventional drug discovery efforts failing to deliver on some critical medical needs, such as non-opioid treatments for chronic pain, there may be value in exploring plants associated with traditional medicines to discover new drug molecules and simplified mixtures. Gb Sciences is connecting plant-based medicine with modern in silico drug discovery tools, leveraging a unique AI-enabled platform to mine plant species and compounds to uncover minimum essential mixtures of molecules with therapeutic benefits and reduced side effect profiles.
To learn more about Gb Sciences, visit www.gbsciences.com.
About Gb Sciences and GbS Global Biopharma
Gb Sciences, Inc. is a plant-inspired, biopharmaceutical research and development company creating patented, disease-targeted formulations of cannabis- and other plant-inspired therapeutic mixtures for the prescription drug market through its Canadian subsidiary, GbS Global Biopharma, Inc. The 'plant-inspired' active ingredients in its therapeutic mixtures are synthetic homologues identical to the original plant compounds but produced under current Good Manufacturing Practices. Gb Sciences' intellectual property portfolio contains six issued U.S. and three issued foreign patents, as well as 17 U.S. and 51 foreign patent-pending applications. In its drug development pipeline, Gb Sciences has five preclinical phase product development programs. Gb Sciences' lead program for Parkinson's disease is being prepared for a first-in-human clinical trial. Gb Sciences' formulations for chronic pain, anxiety, and depression are currently in preclinical animal studies with researchers at the National Research Council Canada. The company also received positive preclinical proof-of-concept data supporting its complex mixtures for the treatment of Cytokine Release Syndrome related to COVID-19, and its lead candidates will be optimized based on late-stage preclinical studies at Michigan State University. Gb Sciences' productive research and development network includes distinguished universities, hospitals, and Contract Research Organizations. To learn more, visit www.gbsciences.com.
Forward-Looking Statements
This press release may contain statements relating to future results or events, which are forward-looking statements. Words such as "expects", "intends", "plans", "may", "could", "should", "anticipates", "likely", "believes" and words of similar import may identify forward-looking statements. These statements are not historical facts, but instead represent only the Company's belief regarding future events, many of which, by their nature, are inherently uncertain and outside of the Company's control. It is possible that the Company's actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward-looking statements. Further, information concerning the Company and its business, including factors that potentially could materially affect the Company's business and financial and other results, are contained in the Company's filings with the Securities and Exchange Commission, available at www.sec.gov. All forward-looking statements included in this press release are made only as of the date of this press release, and we do not undertake any obligation to publicly update or correct any forward-looking statements to reflect events or circumstances that subsequently occur or of which we hereafter become aware.
Media Contact
Alexis Quintal
[email protected]
Press Release Service by Newswire.com
Original Source: TODAY: Gb Sciences Describes Drug Development Progress in Pharma's Almanac Journal Article
George T. ‘Buddy’ Hickman Joins First Health Advisory
WASHINGTON - September 6, 2022 - (Newswire.com)
First Health Advisory, a leading healthcare managed security and technology firm, announced that George T. "Buddy" Hickman will join the organization as Chief Strategy Officer. In this role, Hickman will be central to First Health's strategy formulation and execution. As the healthcare landscape evolves, Hickman will guide First Health to orient imperatives while assuring agile creation of secure and efficient solutions. Hickman will also serve in a customer-facing role, deepening strategic advisory know-how in support of First Health's Enterprise Security & Technology service line.
"I'm thrilled to share the news of Buddy joining First Health. As a former client, advisor, board colleague, and great friend, Buddy has had an outsized influence on our team and the industry as a whole," says Carter Groome, CEO at First Health Advisory. "As healthcare's digital agenda evolves at a torrent pace, Buddy's understanding of how to position and serve the needs of our clients will be instrumental to delivering value to organizations who place their trust in our firm."
In response to joining the First Health team, Buddy noted: "Having the unique experience of having First Health Advisory serve as my trusted business partner, then getting an insider's look while serving as its consulting advisor, my decision to join is well-informed by my having stood with a team that is consistently and highly competent, authentic and values-based. I'm delighted to join this superlative firm to the further benefit our clients."
Hickman brings a wealth of industry experience, knowledge, and his highly regarded reputation to First Health. In his tenured 30 years, Hickman has led large academic health sciences systems as CIO and provided health provider consulting leadership with E&Y and PWC across the U.S. and abroad. He has been recognized as the College of Healthcare Information Management Executives (CHIME)/Healthcare Information and Management Systems Society (HIMSS) John E. Gall, Jr. CIO of the Year. Hickman chaired the boards of both CHIME and HIMSS, and has served on numerous health industry and advisory boards including MedAllies, Health Information Exchange of New York (Hixny), MobileSmith Health, KLAS, and Better Healthcare for Northeast New York. First Health is honored to welcome Buddy Hickman as its Chief Strategy Officer.
About First Health Advisory: First Health Advisory is passionate about securing digital transformation in the healthcare domain. Experts in managed security and technology solutions, First Health Advisory collaborates with customers to safeguard their assets, preserve reputations, and protect balance sheets. Our advisors and programs derive value from investments in technology with the understanding that people have the most significant impact on an organization's risk posture and its alignment with the goals of the business. Please contact [email protected] or visit www.firsthealthadvisory.com for more information.
Related Files
Hickman Press Release_9.6.2022 FINAL.docx
Press Release Service by Newswire.com
Original Source: George T. 'Buddy' Hickman Joins First Health Advisory
MedicalMine Inc. Launches Automatic Insurance Card Reader Using Optical Character Recognition (OCR) Technology in...
South Korea’s CD BIO Seeks Clinical Trial, FDA Registration for Its VOCs Biosensing Kit...
LOS ANGELES - September 5, 2022 - (Newswire.com)
Korean technicians announced on the 30th that they have developed a diagnostic device that diagnoses lung cancer from breathing tests. They have plans to submit the device for U.S. FDA approval and conduct further clinical trials.
To reduce the death rate that is increasing due to cancer, Korean company CD BIO's CEO, Baek Kyung-jin, has developed a diagnostic device VOCs Biosensing Kit that detects lung cancer and various cancers at an early stage with a simple breathing test.
Globalian, a company that opens up export opportunities and conducts export voucher programs with support from the Korean Academy of Clinical Health and the Korean government, has initiated FDA registration and the clinical trial process for CD BIO's VOCs Biosensing Kit through an export voucher program, collaborating with International Licensing Research Solutions, LLC (ILRS) and American Technology Clinical Solutions, Inc. (ATCS). ILRS and ATCS are collaborating, acting as U.S. agents for CD BIO, overseeing all aspects of international marketing.
The clinical trial on the effectiveness of detecting lung cancer and various cancers at an early stage with a simple breathing test was successful, and the thesis presentation was conducted. Now, the company is preparing for a clinical trial with a credible institution for follow-up tests, and at the same time planning to submit for FDA review in the United States in order to advance in the markets in the United States.
CD BIO's CEO, Baek Kyung-jin, said, "Through this clinical trial and the U.S. FDA [review] process, we will play the role of a tool to save people by combining the talents that God has given us with technology. It is the path that I have been praying for until now, and I believe that he will lead me in the future."
Next, he thanked all the technical staff and researchers who participated in the development and said that he will obtain the sober judgment and clear technology direction of the FDA.
Catherine Young
[email protected]
Press Release Service by Newswire.com
Original Source: South Korea's CD BIO Seeks Clinical Trial, FDA Registration for Its VOCs Biosensing Kit That Detects Early Stage Lung and Other Cancers With Simple Breathing Test
neurocare group AG expands international footprint with cooperation in the Middle East, the Republic...
The Worlds Most Expensive Bentley on Reality TV Show
 Debbie Wingham, world renowned for creating countless of the world’s most expensive things herself, recently landed her own reality show on Gossip Stone TV, a media company producing several reality TV shows featuring celebrities, glitz, and glamour. To date Wingham has created 13 one-of-a-kind elaborate commissions, most of which also carry the title of being […]
Debbie Wingham, world renowned for creating countless of the world’s most expensive things herself, recently landed her own reality show on Gossip Stone TV, a media company producing several reality TV shows featuring celebrities, glitz, and glamour. To date Wingham has created 13 one-of-a-kind elaborate commissions, most of which also carry the title of being […] Jennifer Banmiller and Dr Tsai Release Groundbreaking Book: Statutory Vape
SAN FRANCISCO - September 2, 2022 - (Newswire.com)
Jennifer Banmiller, pro consumer advocate and owner of publishing company Periscope Group, and Dr. Tsai, renowned thoracic surgeon, have released an alarming and awakening book on the crisis that is vaping. What they uncovered was a horrific public health nightmare for American youth.
The purpose of this book is to expose this industry for what it is: an unconscionable business that makes billions by selling addictive and potentially fatal products to impressionable youngsters.
The authors hope to shine a spotlight on their insidious plan to hide the dangers of vaping from unsuspecting youth and the "irredeemably evil" fact documented in this book showing the industry preying on children as young as 8 years old by funding summer camps, paying community churches to distribute theorem material, and targeting veterans groups and other vulnerable populations. Targeting young people thus becomes an annuity for the tobacco industry, at a horrible cost to youth.
What began as a master's thesis for two graduate students ended up as the impetus for one of the most treacherous tricks ever played on the American people and particularly on teens and young adults. Insisting that their product was a smoking cessation product, vaping companies, backed by Big Tobacco companies who saw a way back into the youth smoking market share, created the most enticing and cool E-cigarette products imaginable. The marketing was purposeful. First, through packaging, using high-tech, teen-cool, easily hidden devices called vape pens, reminiscent of Apple products, both colorful and sleek.
No scientific testing was done to protect the public prior to the release of these products. And in the face of all this injury and death, E-cig companies only made minor changes to their products and methods when pressured, and even now, as the CDC declares a national vaping epidemic and every state in the U.S. has enacted laws to protect citizens, and over 60 people have died and thousands are permanently injured, these companies continue to profit.
In Statutory Vape, readers will discover how easy it actually was to perpetrate such a dangerous product, about how a simple design supposedly created for one use became a public health hazard once dollars were the prize, about the lack of scientific testing prior to the release of vapes, and about what is being done now so this never happens again.
To learn more about this national crisis and buy the book, please visit statutoryvape.com or periscopegroup.com
For more information on Jennifer Banmiller, visit Jennifer Stanich Banmiller or email [email protected]
Podcast with Dr. Tsai
Related Files
Scannable Document on Sep 1, 2022 at 1_41_13 PM copy.png
Press Release Service by Newswire.com
Original Source: Jennifer Banmiller and Dr Tsai Release Groundbreaking Book: Statutory Vape